
MEDX Xelerator is a leading, medical-device-focused incubator and investor operating under the auspices of the Israel Innovation Authority. Current partners and collaborators include Boston Scientific, Sheba Medical Center, West Pharmaceuticals, MEDX Xelerator Partners, Consensus Business Group (CBG), Wolfson Hospital, Weill Cornell Medicine Enterprise Innovation and Ichilov Hospital.
MEDX Xelerator’s core mission is to identify and nurture high impact medical ventures that fill major unmet clinical needs with large markets to benefit patients, physicians, and investors.
MEDX Xelerator’s management team, comprised of medtech industry veterans, works closely with the incubator’s portfolio companies to consistently achieve outstanding clinical and business milestones in a capital efficient way.
Our incubator continuously scouts for innovative medical technologies in the following areas: vascular, interventional cardiology, urology, interventional oncology, medical robotics and home- and clinic-based digital solutions addressing a sound clinical need that enables remote therapy.
Our vision is to leverage the extraordinary Israeli medtech innovation ecosystem to create breakthrough solutions that genuinely fill unmet medical needs.
Located in Or Yehuda and Sakhnin, Israel, we are an inclusive and diverse team open to all entrepreneurs and researchers eager to advance medical science for the benefit of all.
Download the areas of interest
Strategy
MEDX Xelerator is committed to your success during the incubator period and beyond as you develop and commercialize your medical product. Driven by the unique combination of its management, partners, collaborators, and extensive network, MEDX Xelerator provides the ultimate foundation to any aspiring medical device start up or entrepreneur.
- R & D
- Business Development
- Finance
-
Regulatory
Affairs - Marketing & Sales
- Reimbursement
- IP
- Clinical
Portfolio


SOLUTIONS FOR UNMET NEEDS
If you identify a problem or recognize a need – let us know. We will pull in our resources, and help your idea become reality; from world class engineers through leading clinicians to market makers. Leveraging our innovation generation expertise, together we will find the best solution.
Team
-

Shai Policker
Chief Executive Officer -

Gal Atarot
Chief Technology Officer -

Noam Taichler
Chief Financial Officer -

Soli Cohen-Dahan
HR and Administrative Manager -

Orit Halevy
Accountant -

Noam Gazenfeld
Business Development Manager -

Marjie Hadad
PR General Manager -

Helen Shub
Director of Finance -

Eran Cohen
Director of Business Development -

Inbar Grebler
Business Development Manager
BOARD
-

Harel Gadot
Chairman, CEO and Company Group Chairman MEDX Ventures -

Shai Policker
Chief Executive Officer, MEDX Xelerator -

Eyal Zimlichman, MD
Deputy Director, Chief Medical Officer and Chief Innovation Officer at Sheba -

Elad Duschak
Strategic Advisor to CBG, Healthcare -

Rotem Yehuda Kakon
Strategic Investor & Portfolio Manager Advisor at CBG -

Jonathan Goldstein
Director, Integration and Innovation Strategy -

Avner Halperin
CEO of Sheba Medical Center’s Innovation & Commercialization -

Raphi Levy
Executive Director in Healthcare Banking
Advisors
-

David Bomback, MD
Western Connecticut Health -

Barry T. Katzen MD, FACR, FACC, FSIR
University of Miami School of Medicine -

Menaheam Neuman, MD
Narhariya Hospital -

Christopher Macomber, MD
UMass Memorial -

Lindsay Machan, MD
FRCPC -

Eyal Morag, MD
Assuta Ashdod Medical Center -

Chaim Lotan, M.D., F.A.C.C., F.E.S.C.
Hadassah-Hebrew University Medical Center in Jerusalem -
Professor Charles H Knowles MBBChir, PhD, FRCS, FACCRS (hon)
-

Dave Knapp
Vice President, Corporate Research, Boston Scientific Corporation
Partners
-

Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for more than 35 years,we advance science for life…Read More »
-

CBG is a British investment group with an international investment portfolio in various areas such as pharma, biotech, MedTech, cleantech, real estate and more. CBG’s investment approach driven by an in – depth analysis of trends…Read More »
-

MEDX Ventures Group, founded in 2010, is an investment and management firm with offices in Israel, USA and Europe. The company believes in active management (vs. passive investment) and is focused on medical ventures and technologies…Read More »
-

The Sheba Medical Center at Tel Hashomer is a university-affiliated tertiary referral hospital that serves as Israel’s national medical center in many fields. Adjacent to Tel Aviv, it is the most comprehensive medical center in the Middle East, renowned for…Read More »
-

Intellectual Ventures is a global inventions company. Founded in 2000, we provide the world’s most innovative companies with access to valuable patents and invention related services while creating a wide range of technology-focused partnerships and wholly new companies.Read More »

Recent posts
Roadmap for Entrepreneurs
Do you have an idea for a medical device related to minimally invasive procedures,
medical robotics, medical implants, or digital health?
Here is what to do and an estimated timeline, assuming all approvals are granted along the way.
Click or roll over the stages below for more details:
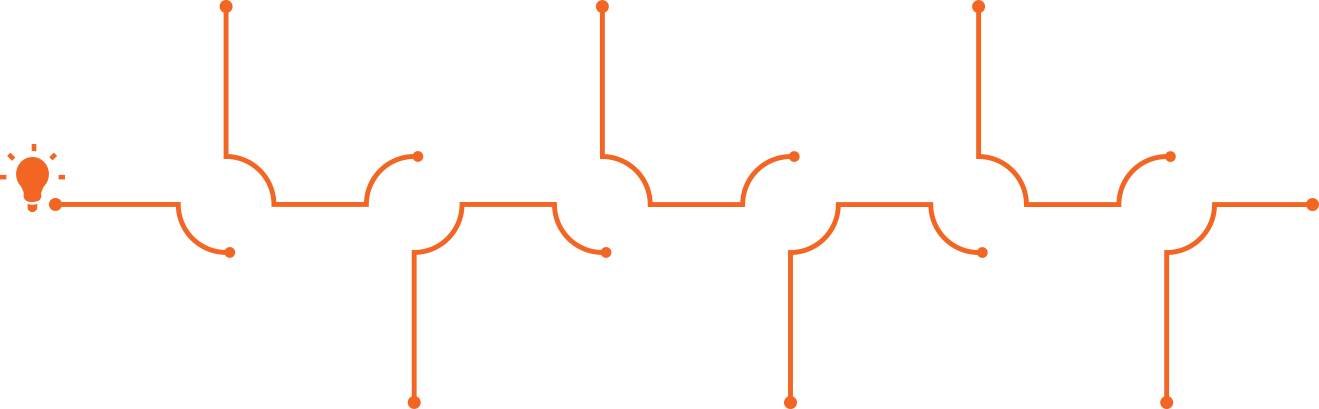
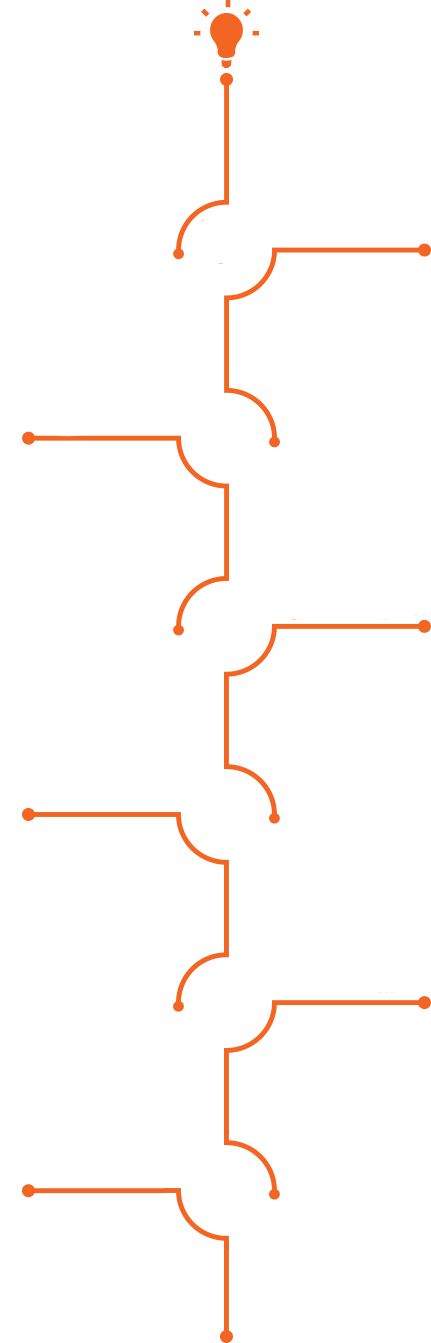
A. Ongoing support from the MEDX Xelerator team and its partners
B. Over $800,000 for a period of 2 years and up to $1.1 million for a period of 3 years
Careers
Trilio Mechanical R&D Engineer
+ Read MorePylon Medical Mechanical Engineer
+ Read MorePortfolio Company CEO Positions
+ Read More
CONTACT US
P.O. Box 619
8 Ariel Sharon St.
Or Yehuda 6037606, Israel































